hcooch ch2 h2o: Small Molecules, Big Health Impact
When you see something like hcooch ch2 h2o, it might look like a secret code from a sci-fi movie. But don’t let the letters fool you these are actually very simple molecules with surprisingly powerful effects on health and everyday life. Today, let’s break down what these small molecules really are, how they work together, and why they matter more than you might think.
What Is hcooch ch2 h2o
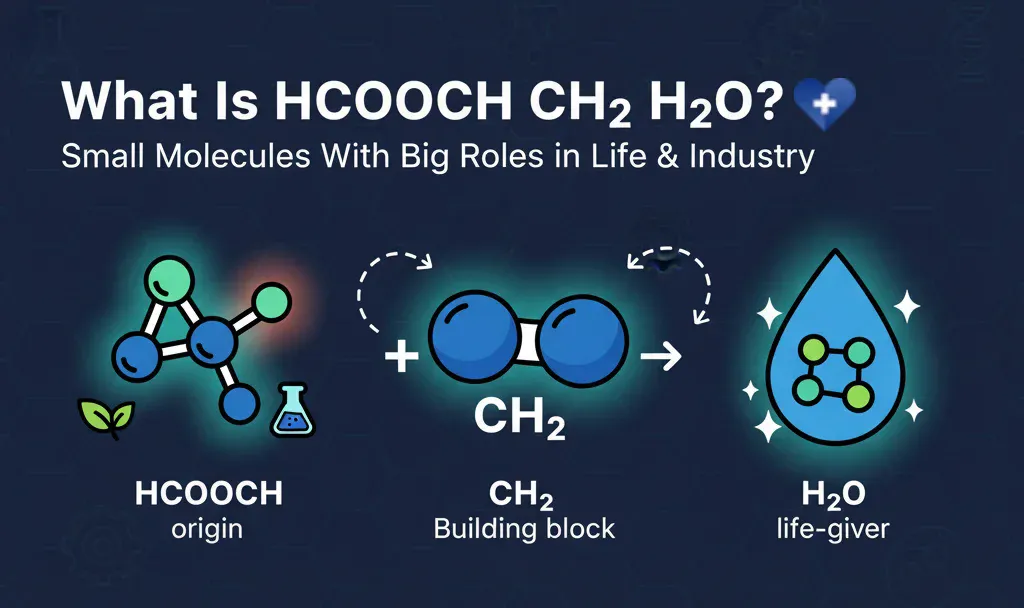
At its core, this formula is a combination of three key players:
- hcooch: This represents methyl formate, an ester formed from formic acid (hcooch) and methanol, often involved in organic reactions.
- ch2: The methylene group, a simple hydrocarbon fragment critical in building bigger molecules, including those in our bodies.
- h2o: Good old water, the life-giver that’s part of almost every chemical and biological reaction on Earth.
Put these together, and you get a small molecular team that pops up in chemistry labs, industry, and even inside living cells. But don’t worry, you don’t need a chemistry degree to understand why they matter.
Why Small Molecules Like These Matter for Health
It’s easy to assume only big, complex molecules like proteins or DNA matter for health. But these small compounds play huge supporting roles behind the scenes.
- Formic Acid and Methyl Formate Nature’s Powerhouses: Formic acid is naturally found in ants (hence the name, from the Latin formica) and is a mild antibacterial agent. It helps defend insects against predators. In humans, formic acid derivatives have been studied for their role in metabolism and as building blocks for pharmaceuticals. Methyl formate, derived from formic acid, finds use in medicines and fragrances, often with low toxicity compared to other industrial solvents.
- Methylene Groups The Molecular Lego Bricks: ch2 isn’t just a formula; it’s the foundation of many organic molecules. It’s in fats, hormones, vitamins, and even the structure of DNA bases. These tiny carbon fragments help form the chains and rings that make up life’s complex molecules.
- Water The Universal Health Medium: Water isn’t just for drinking. At the molecular level, it’s the medium where countless biological reactions happen. It helps dissolve nutrients, carries molecules inside cells, and participates directly in digestion and detoxification processes.
Together, this trio showcases how biology and chemistry intertwine. These small molecules aren’t just lab curiosities they’re essential actors in health wellness, and life itself.
The Chemistry Behind Their Interaction
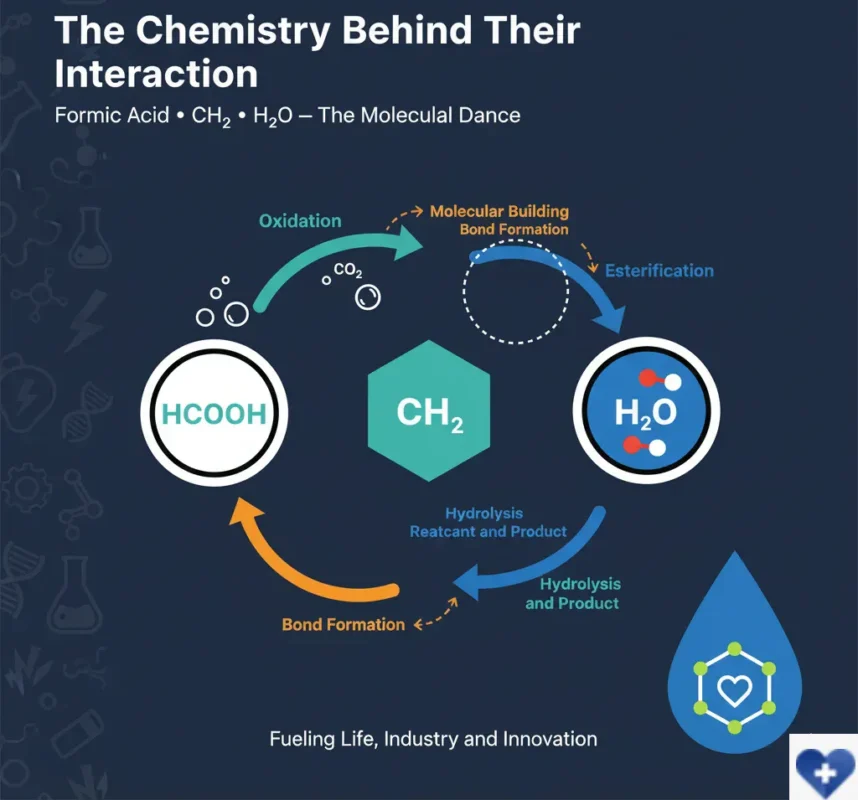
Let’s step into the lab (metaphorically) and see how these molecules play together:
- Reactions in Action: Formic acid (or its esters, like methyl formate) can undergo oxidation to produce carbon dioxide and water a reaction important in metabolic processes. Meanwhile, the ch2 groups often form bonds with other atoms, building the complex molecules that our bodies rely on every second.
- Water as a Reactant and Product: Water not only dissolves these chemicals but also takes part in reactions breaking chemical bonds (hydrolysis) or combining to form new compounds. For example, esterification reactions use water both as a byproduct and as a key player.
These chemical dances allow for endless molecular creativity fueling life, industrial processes, and even pharmaceutical breakthroughs.
Real World Health and Industrial Applications
You might wonder, “Great chemistry, but how does this touch my health or the environment” Here’s the scoop:
- Pharmaceutical Use
Solvents containing methyl formate and water improve the extraction and purification of active ingredients from plants and other sources. This means better medicines, often created with fewer harmful chemicals. - Green Chemistry
These molecules support eco-friendly processes. Using water-based solvents and mild acids like formic acid reduces the need for toxic chemicals in manufacturing, helping protect workers and the planet. - Antibacterial and Preservative Effects
Formic acid and its derivatives have natural antibacterial properties, which can maintain hygiene in cosmetic or food products, indirectly supporting health by reducing harmful bacteria. - Cleaning and Degreasing
There’s an environmental benefit here too: solutions of methyl formate and water are effective in industrial cleaning, breaking down oils and greases without harsh chemicals that pollute water systems.
hcooch ch2 h2o at a Glance: Science Meets Practicality
| Aspect | Science Speak | Why It Matters to You |
|---|---|---|
| Formic acid (hcooch) | Simplest carboxylic acid; reacts easily | Natural antibacterial, less toxic |
| Methylene group (ch2) | Backbone unit in organic molecule chains | Found in fats, vitamins, DNA |
| Water (h2o) | Universal solvent and participant | Essential for all life processes |
| Chemical reactions | Oxidation, hydrolysis, esterification | Basis of metabolism and drug synthesis |
| Industrial use | Green solvent, extraction, cleaning | Safer for environment and workers |
| Health impact | Antibacterial, supports drug purification | Cleaner products, better health |
Safety and Sustainability: Handling with Care and Respect
Even though these molecules look friendly, they need respect:
- Formic acid can be corrosive direct skin contact or inhalation should be avoided.
- Water and methylene compounds are generally safer but can influence how reactive other chemicals are in mixtures.
- Industry uses strict safety protocols to ensure these chemicals benefit us without causing harm or pollution.
On sustainability, water-based solvents and mild acids are a step toward greener chemistry, emphasizing recycling and reducing hazardous waste.
FAQs
Q: Is this molecule found naturally in our bodies?
A: Not exactly the combined form, but its parts, like methylene groups and water, are everywhere in biological molecules.
Q: Can I be exposed to formic acid in everyday life?
A: Formic acid occurs naturally in some insect stings and is used in small amounts in products. Industrial exposure is controlled for safety.
Q: Are these chemicals safe in medicines?
A: Yes, when used properly in controlled amounts, they help make drugs more effective and purer.
Q: Why is water included in the formula?
A: Because it participates actively in reactions and serves as a solvent, making the chemistry possible.
Q: How does this relate to “green chemistry”?
A: Using water and mild, low-toxicity compounds supports eco-friendly manufacturing that’s safer for people and the planet.
Conclusion
From the lab bench to the pharmacy shelf, the humble trio represented by hcooch ch2 h2o leaves a huge footprint. These small molecules formic acid derivatives, methylene fragments, and water are unsung heroes supporting life’s complex chemistry. They make medicines better, industries greener, and products safer.
